1 Unit I – Introduction and Context for the Unit
Interdisciplinary questions we want to answer in this unit
The motivation for this unit is pretty straightforward: you know from Chemistry electrons and photons can behave as both particles and wave a property known as .
- What does this wave-particle duality actually mean?
- WHY does what you learned in chemistry actually work the way it does?
- How does this wave-particle duality result in some of the most fundamental properties of chemistry such as discrete energy levels in atoms?
- Why is the wavelength of a photon emitted from an atom
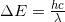 ?
?
Introduction to the Unit
In this unit, we will follow our ontological framework and begin exploring what light and electrons are by listing some of their basic properties. One of the most famous properties of both light and electrons is known as wave-particle duality: sometimes they behave like particles and sometimes they behave like waves. However, electrons and light are neither particles nor waves: they are a completely new type of object with properties of both. This duality is a reflection of the fact that both light and electrons do not obey the laws of Classical Physics that you learned in Physics 131, they are too small. Instead, electrons and light obey Quantum Mechanics. The way I would recommend that you think about the relationship between classical physics and quantum mechanics is in the paradigm of physics striving for ever-more-accurate approximations to reality. Classical mechanics is a good enough approximation to get people to the moon (they did it!). When things get small, you need a better approximation: quantum mechanics. There is a principle, the correspondence principle, which states the quantum mechanics must reproduce classical mechanics for large objects.
In this unit we will explore both the wave and particle natures of light and electrons. First however, we must define to ourselves what waves and particles are! Following our ontological framework, we will therefore need to look at some of the basic properties that characterize waves and particles in general. Thus, we will begin with some review of particles from physics 131 and then a discussion of waves, which may be familiar to some of you. Once we have defined waves and particles through listing their properties we will explore how these properties manifest for electrons and light.
As you read, you MUST keep in mind that light and electrons are neither particles nor waves. They are something completely new (quantum mechanical objects) that you have zero previous experience with. Particles and waves are simply ways of visualizing these objects in ways our brains can understand. Neither picture is 100% correct. The correct approach is to jump back-and-forth between these two pictures
What we will do in class
This idea of electrons and light being neither particles no waves but having properties of both is a hard one to get used to. In class, we will spend a lot of time practicing jumping between the wave and particle pictures: seeing the benefits that each picture can bring in various situations. The goal is that, through practice, you become more comfortable with bouncing back and forth between pictures as the situation demands.
After a bit of practice with moving between the wave picture and particle pictures of light and electrons, we will combine this understanding with one of the most important ideas in physics: conservation of energy. Thinking about this fundamental principle in conjunction with the fundamentally quantum nature of light and electrons will allow us to understand many different phenomena such as, “Why do electrons in atoms have defined energy levels?” You know from chemistry courses that they do. Our goal is to explain why!
What you should get out of this prep
In order to explore these ideas in class, you need to have a grasp on the basic terminology of waves and particles. You need to know that particles are characterized by their energy, momentum, and number while waves are described by their wavelength and frequency/period, and amplitude. Both waves and particles can be characterized by a speed: a critical fact for converting between the wave and particle pictures. The following chapters will refresh energy and momentum from 131 and introduce the needed concepts for waves: amplitude, frequency, and wavelength. You need to know what all these terms mean, the basic formulas for them, and how they are connected
Also in this unit, some of the basic properties of light and electrons. We will establish electrons via a tour of the atom (probably review for most of you). We will also introduce anti-matter: a type of matter identical to the normal matter with which you are familiar in every respect with two exceptions. First, anti-matter has the opposite charge from normal matter (anti-electrons have positive charge). Second, when matter and anti-matter collide, the result is light. While there are other interesting questions about anti-matter (why isn’t it everywhere?) those two points are all you need to know from the prep. With regards to light, we will introduce the different kinds of light (radio, infrared, …) and the particle of light: the photon. Some of the basic properties of the photon will be introduced. The most important of which you need for the prep are the fact that the mass of the photon is zero and the fact that it always travels at the speed of light c. Finally, we will also explore de Broglie’s relationship on how to connect wave and particle picture
The last topic in this unit’s preparation you need to be familiar with is the idea of intensity: energy per area per time. The text will introduce the idea of power (energy per time) and its unit the Watt. This concept will also be important for converting between the wave and particle pictures.

Instructor’s Notes
This is a lot of information. Remember, we are not expecting mastery of it all and we are certainly not expecting you to have a complete picture of how everything Just make sure you know the definitions, formulas, and units for everything. To help keep you focused on what is important, there is a summary tables below (only focusing on the facts for your quiz). Keep these tables handy as you go through the reading: the information will all be explained as you go.
Waves and Particles
This information applies to both electrons and light.
Intensity, detailed later, is the energy per time per area (or power ![]() ):
):
![]()
| Properties | Convert between them | |
| Waves |
|
|
| Particles |
|
Electrons and Photons
Note, in general, if you see a ![]() or an
or an ![]() in an equation, it applies to light only! The wave and particle information in the table still applies to both electrons and photons!
in an equation, it applies to light only! The wave and particle information in the table still applies to both electrons and photons!
| Waves | Particles | |
| Light |
|
|
| Electrons |
|
|
A description for the fundamentally new nature of very small objects like electrons and photons: sometimes they behave like waves and sometimes they behave like particles. Neither picture is 100% correct: electrons are neither waves nor particles, but have properties of both.
In fact, all objects exhibit wave particle duality. You have a wavelength! However, your wavelength is too small to notice (check with de Broglie if you want). The effect is really only noticeable for small objects.

