20 Basics of Charge
Static Electricity and Charge
Editors’ note: This section is derived from 18.2 Static Electricity and Charge: Conservation of Charge by OpenStax, Bobby Bailey
What makes plastic wrap cling? Static electricity. Not only are applications of static electricity common these days, but its existence has been known since ancient times. The first record of its effects dates to ancient Greeks, who noted more than 500 years B.C. that polishing amber temporarily enabled it to attract bits of straw (see Figure 1). The very word electric derives from the Greek word for amber (electron).
Many of the characteristics of static electricity can be explored by rubbing things together. Rubbing creates the spark you get from walking across a wool carpet, for example. Static cling generated in a clothes dryer and the attraction of straw to recently polished amber also result from rubbing. Similarly, lightning results from air movements under certain weather conditions. You can also rub a balloon on your hair, and the static electricity created can then make the balloon cling to a wall. We also have to be cautious of static electricity, especially in dry climates. When we pump gasoline, we are warned to discharge ourselves (after sliding across the seat) on a metal surface before grabbing the gas nozzle. Attendants in hospital operating rooms must wear booties with aluminum foil on the bottoms to avoid creating sparks, which may ignite the oxygen being used.
How do we know there are two types of electric charge? When various materials are rubbed together in controlled ways, certain combinations of materials always produce one type of charge on one material and the opposite type on the other. By convention, we call one type of charge “positive,” and the other type “negative.” For example, when glass is rubbed with silk, the glass becomes positively charged and the silk negatively charged. Because the glass and silk have opposite charges, they attract one another like clothes that have rubbed together in a dryer. Two glass rods rubbed with silk in this manner will repel one another, since each rod has positive charge on it. Similarly, two silk cloths so rubbed will repel, since both cloths have negative charge. Figure 2 shows how these simple materials can be used to explore the nature of the force between charges.
More sophisticated questions arise. Where do these charges come from? Can you create or destroy charge? Is there a smallest unit of charge? Exactly how does the force depend on the amount of charge and the distance between charges? Such questions obviously occurred to Benjamin Franklin and other early researchers, and they interest us even today.
Charge Carried by Electrons and Protons
Franklin wrote in his letters and books that he could see the effects of electric charge but did not understand what caused the phenomenon. Today, we have the advantage of knowing that normal matter is made of atoms, and that atoms contain positive and negative charges, usually in equal amounts.
Figure 3 shows a simple model of an atom with negative electrons orbiting its positive nucleus. The nucleus is positive due to the presence of positively charged protons. Nearly all charge in nature is due to electrons and protons, which are two of the three building blocks of most matter. (The third is the neutron, which is neutral, carrying no charge.) Other charge-carrying particles are observed in cosmic rays and nuclear decay, and are created in particle accelerators. All but the electron and proton survive only a short time and are quite rare by comparison.
The charges of electrons and protons are identical in magnitude but opposite in sign. Furthermore, all charged objects in nature are integral multiples of this basic quantity of charge, meaning that all charges are made of combinations of a basic unit of charge. Usually, charges are formed by combinations of electrons and protons. The magnitude of this basic charge is
![]()
The symbol ![]() is commonly used for charge, and the subscript
is commonly used for charge, and the subscript ![]() indicates the charge of a single electron (or proton).
indicates the charge of a single electron (or proton).
The SI unit of charge is the coulomb (C). The number of protons needed to make a charge of 1.00 C is
![]()
Similarly, ![]() electrons have a combined charge of −1.00 coulomb. Just as there is a smallest bit of an element (an atom), there is a smallest bit of charge. There is no directly observed charge smaller than
electrons have a combined charge of −1.00 coulomb. Just as there is a smallest bit of an element (an atom), there is a smallest bit of charge. There is no directly observed charge smaller than ![]() , and all observed charges are integral multiples of
, and all observed charges are integral multiples of ![]() .
.
Figure 4 shows a person touching a Van de Graaff generator and receiving excess positive charge. The expanded view of a hair shows the existence of both types of charges but an excess of positive. The repulsion of these positive like charges causes the strands of hair to repel other strands of hair and to stand up. The further blowup shows an artist’s conception of an electron and a proton perhaps found in an atom in a strand of hair.
Key Takeaways: Things Great and Small: The Submicroscopic Origin of Charge
With the exception of exotic, short-lived particles, all charge in nature is carried by electrons and protons. Electrons carry the charge we have named negative. Protons carry an equal-magnitude charge that we call positive. (See Figure 4.) Electron and proton charges are considered fundamental building blocks because all other charges are integral multiples of those carried by electrons and protons. Electrons and protons are also two of the three fundamental building blocks of ordinary matter. The neutron is the third and has zero total charge.

Instructor’s Note
The fact that there are no observed free particles with less than ![]() of charge is important and will be used in some of your homework problems.
of charge is important and will be used in some of your homework problems.
Separation of Charge in Atoms
Charges in atoms and molecules can be separated—for example, by rubbing materials together. Some atoms and molecules have a greater affinity for electrons than others and will become negatively charged by close contact in rubbing, leaving the other material positively charged. (See Figure 5.) Positive charge can similarly be induced by rubbing. Methods other than rubbing can also separate charges. Batteries, for example, use combinations of substances that interact in such a way as to separate charges. Chemical interactions may transfer negative charge from one substance to the other, making one battery terminal negative and leaving the first one positive.
No charge is actually created or destroyed when charges are separated, as we have been discussing. Rather, existing charges are moved about. In fact, in all situations, the total amount of charge is always constant. This universally obeyed law of nature is called the law of conservation of charge.
Play with the Simulation
Below is a simulation of a balloon and a sweater. As you probably know, if you rub a balloon on a sweater, it will stick to a wall.
A few things to note:
- The total number of charges is conserved – electrons move from the sweater to the balloon.
- If you have two balloons with negative charge, they will repel, just like in real life (check it for real if you don’t believe us!)
- When you bring the balloon near the wall, what happens to the electrons in the wall?
Law of Conservation of Charge
The total charge is constant in any process.
Section Summary
- There are only two types of charge, which we call positive and negative.
- Like charges repel, unlike charges attract, and the force between charges decreases with the square of the distance.
- The vast majority of positive charge in nature is carried by protons, while the vast majority of negative charge is carried by electrons.
- The electric charge of one electron is equal in magnitude and opposite in sign to the charge of one proton.
- An ion is an atom or molecule that has nonzero total charge due to having unequal numbers of electrons and protons.
- The SI unit for charge is the coulomb (C), with protons and electrons having charges of opposite sign but equal magnitude; the magnitude of this basic charge
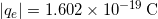
- Whenever charge is created or destroyed, equal amounts of positive and negative are involved.
- Most often, existing charges are separated from neutral objects to obtain some net charge.
- Both positive and negative charges exist in neutral objects and can be separated by rubbing one object with another. For macroscopic objects, negatively charged means an excess of electrons, and positively charged means a depletion of electrons.
- The law of conservation of charge ensures that whenever a charge is created, an equal charge of the opposite sign is created at the same time.

