20 Open Systems and Transferring Energy
Brokk Toggerson
Open Systems
Unlike a , an is one where energy is free to move in or out, being exchanged with the rest of the Universe. Some examples of open systems could include a thrown ball where air resistance is included: the ball loses energy due to friction with the air. Another example could be a chemical reaction which is taking place inside a container which is located on top of an open flame: energy is coming in from the flame to the reactants in the container.
Ways of Transferring Energy: Work and Heat
We classify all of the possible ways add energy to or subtract energy from a system into two main categories: work and heat. We have explored both of these already, but it is worth comparing the two concisely:
- : the transfer of energy via a force applied for some distance.
- : the diffusion of energy from a region of high concentration to one of low concentration.
We can also turn this idea on its head: if the energy in a system changes, then we can infer that either work or heat was present.
More on Work
We have already introduced work in the chapter What Is Energy? Here, we want to explore it in a bit more detail.
Applying a Force for a Distance
Example: Adding or Subtracting Energy
Problem:
Below you see a picture of a book sliding down a wall:
- The gravity pulls the book down.
- The hand pushes the book into the wall.
- The friction fights the downward motion.
For each force, determine if the work is adding or subtracting energy from the system or doing nothing.
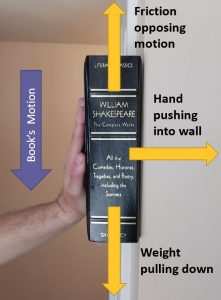
Solution:
a. The gravity pulling the book downwards.
The work done by this force is adding energy into the system as the force is in the same direction as the force’s motion.
b. The hand pushing the book into the wall.
This force is NOT doing any work on the book because it is perpendicular to the motion.
c. The friction opposing the motion.
The work done by this force is subtracting energy from the system as the force is opposing the motion.
Another Way to Think About Work: Pressure and Changes in Volume
Let’s explore another way to think about work. Well the definition of work that we have so far, a push applied for a distance, works really well for objects like pepple, cars and blocks. However, in the life sciences, we are often interested in the behavior of things that physicists call fluids, which means gases and liquids. For gases and liquids, you tend to not be interested in the force you apply to them but instead the .
How will we figure out the expression for work in terms of pressure? Well, we will do this simple example of a gas inside of a container with a piston.
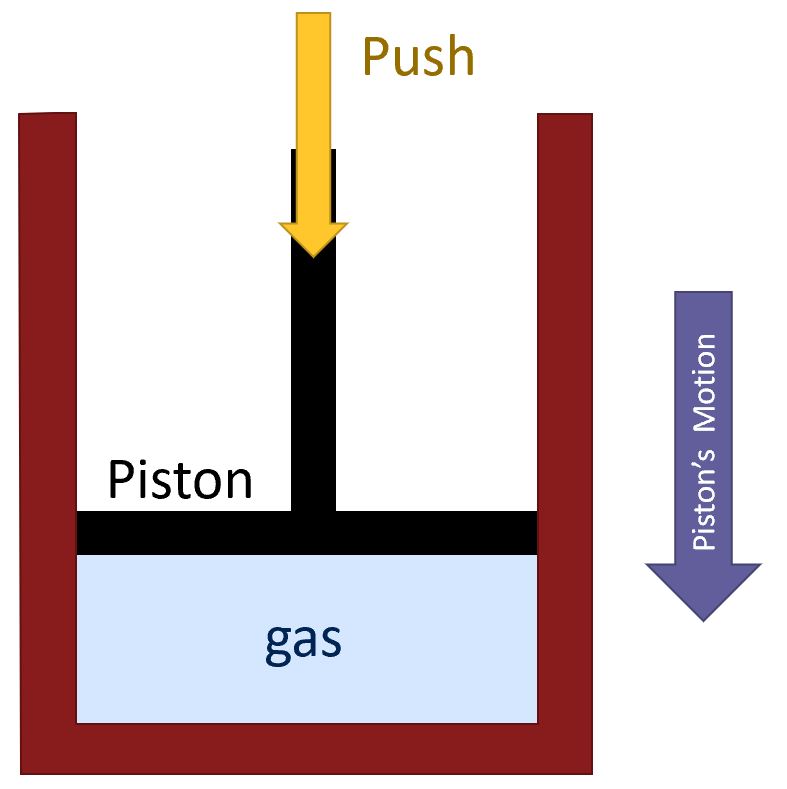
As we compress the gas:
- We are applying a force on the piston for some distance.
- We are doing work on the gas.
- Since the force is in the same direction as the motion, we are adding energy the the system.
Since we want to think about gases more generally, instead of thinking of forces and distances, we should think in terms of quantities more relevant to a gas: pressure and volume:
- The force is spread over the area of the piston, i.e. we can think of a pressure. If we had a smaller piston with the same force, we would have a higher pressure.
- The volume is decreasing.
From here we can conclude that, in addition to a force and a distance, we can think of work in terms of a pressure and a change in volume:
- If the system’s volume is decreasing, energy must be being added.
- By extension,if a system’s volume is expanding, energy my be being subtracted.
Example: Returning to the Marshmallow in the Microwave
Problem:
In the chapter Overview of the Types of Energy, I used a marshmallow in a microwave to describe how temperature is a kinetic energy. Now let’s return to that example in terms of work.
a. Is the air doing work on the marshmallow? If so, is it adding energy in, or subtracting it out?
b. Is the marshmallow doing work on the air? If so, is it adding energy in, or subtracting it out?
c. How do these two amounts of work (air on marshmallow and marshmallow on air) compare?
Solution:
A key thing to think about here the perspective: who is doing work on whom?
a. Is the air doing work on the marshmallow? If, so is it adding energy in, or subtracting it out?
For this part, we want the work done by the air on the marshmallow. The pressure of the air is, 1 atmosphere’s worth ![]() (it is open to the rest of the atmosphere after all!). The volume of the marshmallow (the thing being acted on) is increasing, so energy is leaving the marshmallow and going into the air. The air is still doing work, it is just doing work in the same way as a truck being pushed opposite the direction of motion: in a way that takes energy from the marshmallow.
(it is open to the rest of the atmosphere after all!). The volume of the marshmallow (the thing being acted on) is increasing, so energy is leaving the marshmallow and going into the air. The air is still doing work, it is just doing work in the same way as a truck being pushed opposite the direction of motion: in a way that takes energy from the marshmallow.

b. Is the marshmallow doing work on the air? If so, is it adding energy in, or subtracting it out?
Now we are interested in the work done by the marshmallow on the air. The pressure is still ![]() . In this case, the thing being acted on is the air in the microwave. Since the volume of the air is decreasing (the total volume of the microwave is constant: if the marshmallow gets bigger, the air has to get smaller!), it must be gaining energy: the marshmallow is doing work on the air in a way that adds energy to the air.
. In this case, the thing being acted on is the air in the microwave. Since the volume of the air is decreasing (the total volume of the microwave is constant: if the marshmallow gets bigger, the air has to get smaller!), it must be gaining energy: the marshmallow is doing work on the air in a way that adds energy to the air.
c. How do these two amounts of work (air on marshmallow and marshmallow on air) compare?
Since energy is conserved, the two amounts of work must be the same: the amount of energy taken by the air from the marshmallow must equal the amount given by the marshmallow to the air.
Think of it like money: if ![]() 5) must be the same as the amount you give to me (also
5) must be the same as the amount you give to me (also ![]() 5 went from you to me. The only difference is perspective: did I take it or did you give it?
5 went from you to me. The only difference is perspective: did I take it or did you give it?
Heat
The other way of transferring energy we already talked about in our unit on entropy: heat. As we discussed then, heat is the diffusion of energy from an area of high concentration to an area of low concentration. There are three ways to do this discussed in the video below.
Example: Can an Object Have Heat?
Problem:
Can an object have heat? Why or why not?
Solution:
Heat is the diffusion of energy. As such it is a transfer and an object cannot possess heat.
Conservation of Energy in an Open System
Example: Cooking an Egg
Problem:
Tell me a story of the energy in a egg that is being boiled in a pot of water. How does the chemical potential energy change? What about the thermal energy? Are these changes due to the addition or subtraction of energy via heat and/or work? In the case of heat, is it conduction, convection, or radiation.

Solution:
Heat energy moves from the pan into the water via conduction (the water is in contact with the pan): the fast moving molecules in the pan jostle the water molecules, giving them more kinetic energy and thereby raising their temperature. This energy then diffuses throughout the water via convection. This energy then moves through the shell of the egg via conduction. Once inside the energy causes the proteins to move faster and faster, raising their temperature. As a consequence of all of this moving, the proteins “denature,” i.e. they untangle moving the atoms further and further apart. As the atoms move further apart their potential energy increases (gets less negative / closer to zero).
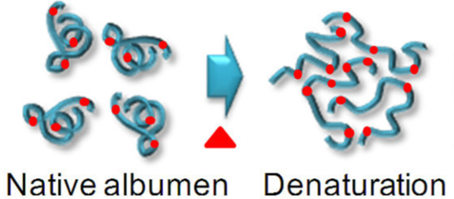
In summary:
- Heat is added to the water from the pot via conduction.
- Heat is distributed through the water via convection.
- Heat is added to the egg from the water via conduction.
- The thermal energy of the egg increases as the egg’s protein molecules go faster and faster (warm).
- The chemical potential energy of the egg’s proteins increases as they denature.
- No work is done as there is no force applied for a distance nor any changes in volume.
Homework
- The ability to do work.
- Ranking boxes with the same force applied.
- Open systems with non-zero heat.
- Change in thermal and chemical potential energy.
A collection of objects where energy cannot enter or leave. It can be exchanged among the objects within the system, but cannot leave, nor can more energy come in.
A collection of objects which is free to exchange energy with its environment.
A force applied for some distance: when I push or pull on an object for some distance, I am doing work on it.
The diffusion of energy from a region of high concentration to a region of low concentration.
How much a force is spread out over an area.

