13 Basics of Matter
Brokk Toggerson and OpenStax
Part of our goal in this unit is to explore energy across different disciplines (biology, chemistry, and physics) as well as across distance scales: from the molecular to the size of the entire planet. To achieve this goal, we need to be familiar with the properties of matter on an atomic/molecular scale. Much of this will be similar to what you discussed in your Chemistry courses, but, since we are approaching it from a physics perspective, it may look a bit different. I would encourage you to reflect on how the material we are presenting here is similar to, but also different from, how the same material was presented in your chemistry/biology courses. You will also be expected to have a familiarity with the basics of the periodic table and how to convert from a mass to a number of molecules/atoms via Avogadro’s number. Please see the relevant appendices if you need a review of this material.
Matter in General
Atoms[1]
The development of modern atomic theory revealed much about the inner structure of atoms. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. The diameter of an atom is on the order of 10−10 m, whereas the diameter of the nucleus is roughly 10−15 m—about 100,000 times smaller. For a perspective about their relative sizes, consider this: If the nucleus were the size of a blueberry, the atom would be about the size of a football stadium (Figure 1).

Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 × 10−23 g, and an electron has a charge of less than 2 × 10−19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). The amu was originally defined based on hydrogen, the lightest element, then later in terms of oxygen. Since 1961, it has been defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. (This isotope is known as “carbon-12” as will be discussed later in this module.) Thus, one amu is exactly ![]() of the mass of one carbon-12 atom: 1 amu = 1.6605 × 10−24 g. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C.
of the mass of one carbon-12 atom: 1 amu = 1.6605 × 10−24 g. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C.
A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu (it would take about 1800 electrons to equal the mass of one proton. The properties of these fundamental particles are summarized in Table 3. (An observant student might notice that the sum of an atom’s subatomic particles does not equal the atom’s actual mass: The total mass of six protons, six neutrons, and six electrons is 12.0993 amu, slightly larger than 12.00 amu. This “missing” mass is known as the mass defect, and you will learn about it in the chapter on nuclear chemistry.)
| Name | Location | Charge (C) | Unit Charge | Mass (amu) | Mass (g) |
|---|---|---|---|---|---|
| electron | outside nucleus | −1.602 × 10−19 | 1− | 0.00055 | 0.00091 × 10−24 |
| proton | nucleus | 1.602 × 10−19 | 1+ | 1.00727 | 1.67262 × 10−24 |
| neutron | nucleus | 0 | 0 | 1.00866 | 1.67493 × 10−24 |
| Table 3. Properties of Subatomic Particles | |||||
The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element: Its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Therefore, the atomic number also indicates the number of electrons in an atom. The total number of protons and neutrons in an atom is called its mass number (A). The number of neutrons is therefore the difference between the mass number and the atomic number: A – Z = number of neutrons.
![Rendered by QuickLaTeX.com \begin{array}{r @ {{}={}} l} \text{atomic number (Z)} & = \text{number of protons} \\[1em] \text{mass number (A)} & = \text{number of protons + number of neutrons} \\[1em] \text{A - Z} & = \text{number of neutrons} \end{array}](https://openbooks.library.umass.edu/toggerson-131/wp-content/ql-cache/quicklatex.com-7ac1ef9b95de41f361d5b177a97e5717_l3.png)
Compounds[2]
Ionic Compounds
When an element composed of atoms that readily lose electrons (a metal) reacts with an element composed of atoms that readily gain electrons (a nonmetal), a transfer of electrons usually occurs, producing ions. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, Na+, and each chlorine atom in a sample of chlorine gas (group 17) accepts one electron to form a chloride anion, Cl−, the resulting compound, NaCl, is composed of sodium ions and chloride ions in the ratio of one Na+ ion for each Cl− ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form CaCl2, which is composed of Ca2+ and Cl− ions in the ratio of one Ca2+ ion to two Cl− ions.
A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the compound is usually ionic. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. However, it is not always true (for example, aluminum chloride, AlCl3, is not ionic).
Molecular Compounds
Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Covalent bonding is an important and extensive concept in chemistry, and it will be treated in considerable detail in a later chapter of this text. We can often identify molecular compounds on the basis of their physical properties. Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist.
The Phases of Matter
Specific Models for Specific States of Matter
Following our concepts of model building, there are two particular models for gases and solids that warrant special attention.
The Ideal Gas
The Einstein Solid
Much of the matter we typically deal with in our everyday lives is a solid. The idea of “solid” is something that retains is shape. This is an idealization — it really means “it doesn’t change its shape while I’m watching it.” As we know, a solid can change its shape if you push on it hard enough (by deforming or breaking). Some things that appear solid will change their shape slowly under the gentle pull of gravity. Some glass in very old windows shows a small amount of sagging. And what is it with butter, anyway?
A uniform solid is one where every part of it is the same as every other part. This is a model statement, since all matter is composed of atoms and at the atomic level no matter is uniform since there are spaces between the atoms. When we say something is a “uniform solid” we mean that we are going to be examining it on a scale large enough that we can ignore atomic discontinuities.
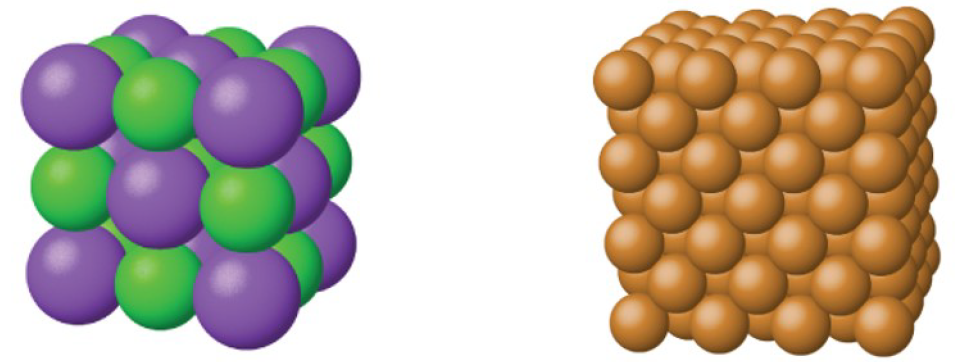
Some uniform solids consist of atoms or ions in a regular pattern, such as diamonds composed only of carbon atoms in a regular lattice, or a salt crystal composed of sodium and chloride ions in a regular array. However, these are relatively uncommon and so for the most part we will consider solids to made up of molecules. Solids can be formed from a single compound where all the molecules are the same, or multiple compounds, with a mixture of molecules. A multi-component solid is sometimes harder to characterize as it can be a non-uniform mixture or conglomerate.
Biological solids can be quite complex. They are often not uniform, but instead are composites, made of particles embedded in layers composed of different materials. Your skin is a good example. It is composed of a thick layer of connective tissue (dermis) underlain by a membrane and overlaid by a surface (epidermal) layer. The dermis is actually a three dimensional network of collagen fibers that are embedded in a protein-polysaccaride matrix. The stretchiness of your skin is provided by additional elastin fibers which are distributed throughout the dermis.
When most liquids are cooled, they eventually freeze and form crystalline solids, solids in which the atoms, ions, or molecules are arranged in a definite repeating pattern. It is also possible for a liquid to freeze before its molecules become arranged in an orderly pattern. The resulting materials are called amorphous solids or noncrystalline solids (or, sometimes, glasses). The particles of such solids lack an ordered internal structure and are randomly arranged.
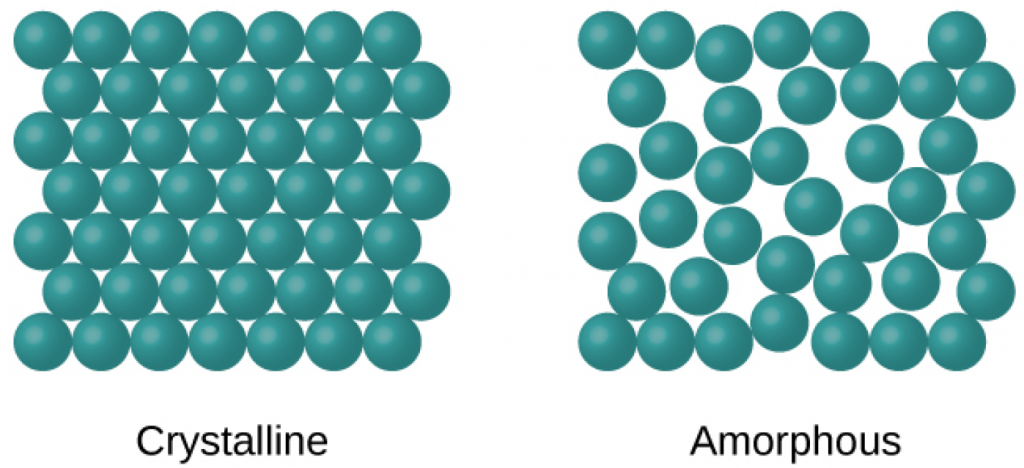
Metals and ionic compounds typically form ordered, crystalline solids. Substances that consist of large molecules, or a mixture of molecules whose movements are more restricted, often form amorphous solids. For examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. Some substances, such as boron oxide (shown in Figure 1.21), can form either crystalline or amorphous solids, depending on the conditions under which it is produced. Also, amorphous solids may undergo a transition to the crystalline state under appropriate conditions.
A crystalline solid, has a precise melting temperature because each atom or molecule of the same type is held in place with the same forces or energy. Thus, the attractions between the units that make up the crystal all have the same strength and all require the same amount of energy to be broken. The gradual softening of an amorphous material differs dramatically from the distinct melting of a crystalline solid. This results from the structural nonequivalence of the molecules in the amorphous solid. Some forces are weaker than others, and when an amorphous material is heated, the weakest intermolecular attractions break first. As the temperature is increased further, the stronger attractions are broken. Thus amorphous materials soften over a range of temperatures.
In this class, we will be focused on a model called the Einstein solid model of simple crystalline solids, particularly those where the atoms are arranged in simple cube-based structures such as table salt (NaCl) and copper as shown in Figure 10. This model, named after Albert Einstein, is like the ideal gas law discussed in the previous section: almost no solid behaves exactly like an Einstein solid. However, the behavior of many solids is approximately Einsteinian and the principles behind the model can be used successfully to understand the more complex solids that you will probably study in your other science courses.
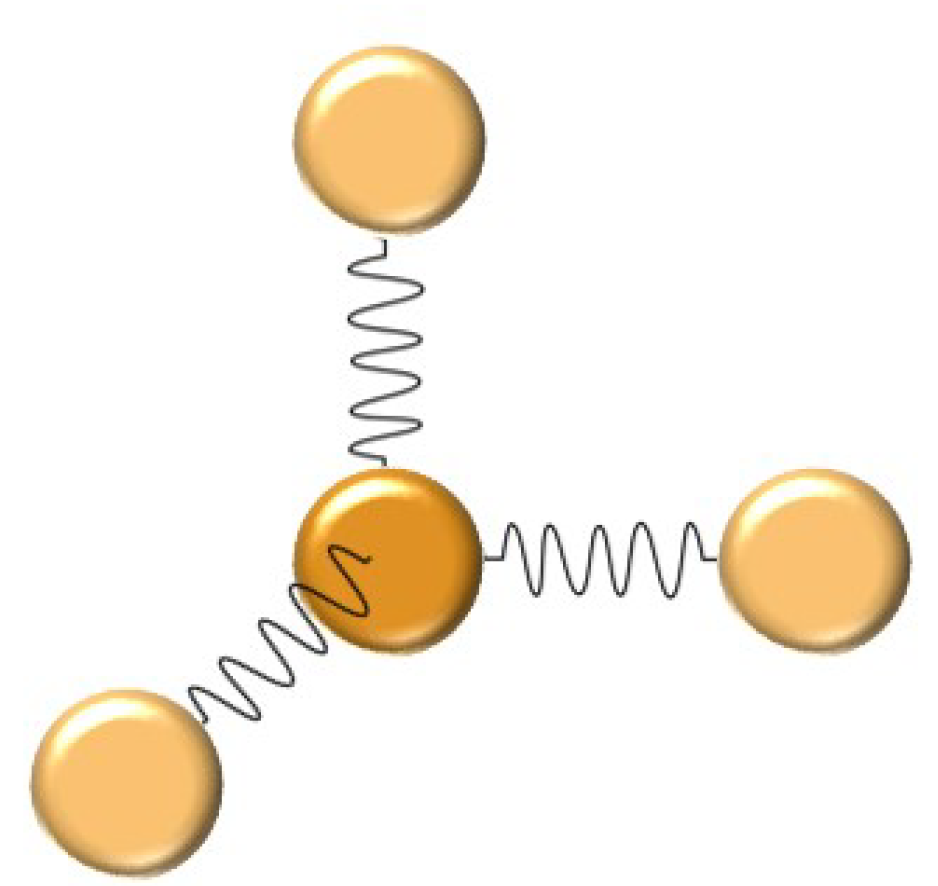
In the Einstein solid model, shown in the figure below, we model the bonds between each atom as tiny springs. While chemical bonds, like everything else, do NOT exactly behave like springs, the modelling of atomic bonds as springs is very common throughout physics, chemistry, and biology. The reason this model is so common is that, for small shifts, atoms do behave as if they are on springs: pull two atoms in a crystal apart and the atomic bonds will pull them back together. If you push two atoms too close together, then the two positively charged nuclei will repel each other pushing them apart. The result is that each atom vibrates about its equilibrium position or lattice site as if it were attached to tiny springs. Given that calculations with Hooke’s Law are so simple to do in comparison to full calculations of atomic forces, you can see why this Einstein model is a useful approximation.
Homework
- Phases/states of matter.

